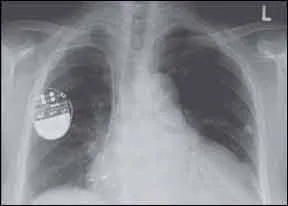
Hearing that a safety advisory has been issued for your implantable cardioverter-defibrillator (ICD) could be a frightening piece of news. But a recent study by Cleveland Clinic researchers suggests that an advisory from the Food and Drug Administration (FDA) is not associated with a significantly higher mortality rate than what would otherwise be expected in a patient with such a device. In the study, published in a recent issue of HeartRhythm, the mortality rate of people who whose devices were never the subject of an advisory was 52 percent over a period of 5.5 years, while the mortality rates of those whose devices were subject to a Class I advisory by the FDA was 54.1 percent, and 43.6 percent in patients with devices that received Class II advisories. The FDA divides medical devices into three classes: Class I and II devices are generally considered lower-risk, while Class III devices are higher risk and/or first-of-a-kind items.
To continue reading this article or issue you must be a paid subscriber.
Sign in