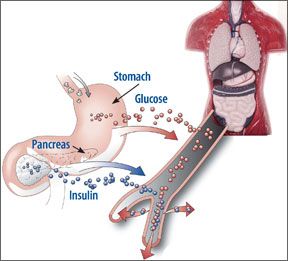
The diabetes drug rosiglitazone (Avandia) has been in the headlines in recent months, having earned a less-than-stellar vote of confidence from a Food and Drug Administration advisory panel that voted 20-12 to keep the medication on the shelf, but with severe restrictions and warnings, as well as further review of its safety. Rosiglitazone has come under fire in recent years after studies have linked it to a higher risk of heart attacks and heart disease. At press time, Cardiologist Steven Nissen, MD, an outspoken critic of the drug and the chairman of the Department of Cardiovascular Medicine at Cleveland Clinic, says he expects the FDA will likely ban rosiglitazone.
To continue reading this article or issue you must be a paid subscriber.
Sign in